DNA-protein crosslinks: endogenous origins and cellular responses
Our work addresses the most pressing questions in the emerging field of research on DNA-protein crosslinks (DPCs) and their repair. Covalent DPCs are highly toxic, because they block chromatin processes such as transcription and replication. DPCs are induced by various endogenous and exogenous agents (e.g. reactive aldehydes) and are also caused by covalent trapping of enzymes such as topoisomerases. Until recently, it was assumed that these lesions were simply targeted by canonical DNA repair pathways. This has changed with our discovery of a protease-based DPC-specific repair mechanism, which is conserved from yeast to humans. Our work established that proteases of the SPRTN family employ a unique DNA-dependent activity to degrade the protein components of DPCs, which maintains genome stability and ensures tumour suppression. Strikingly, DPC repair by SPRTN is essential for cellular viability, which suggests that chromatin is constantly challenged with substantial amounts of endogenous DPCs. Our data indicates that DPCs are key drivers of genome instability and that there is an entire unexplored pathway regulating protease-based DPC repair. The overarching objective of our work is thus to reveal the sources of endogenous DPCs and to understand the cellular responses to these threats not only in mechanistic detail but also on a global scale.
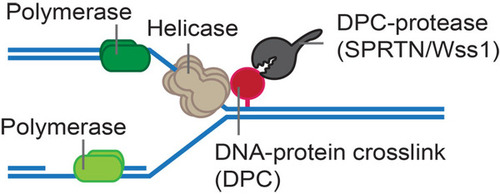
Figure legend: DPCs are highly toxic because they block replication. DPC proteases (Wss1 in yeast, SPRTN in higher eukaryotes) can degreade the protein component of DPCs, thereby allowing replication to progress.


